NEW YORK — U.S. health officials on Wednesday recommended that people 50 and older get a shot against bacteria that can cause pneumonia and other dangerous illnesses.
The recommendation was made by a scientific advisory panel and then accepted by the Centers for Disease Control and Prevention. The decision lowered from 65 the minimum recommended age for adults to get the shot.
TODAY: Second dose of COVID vaccine recommended for ages 65 and older
The advisory committee voted 14-1 to make the change during a meeting in Atlanta. The guidance is widely heeded by doctors and prompts health insurers to pay for recommended shots.
Pneumococcal shot recommendations are sometimes called the most complicated vaccination guidance that the government issues. The CDC currently recommends shots for children younger than 5 and adults 65 or older, as long as they have never been vaccinated against pneumococcal disease. Officials also recommend the shots for children and adults at increased risk for pneumococcal disease, such as those with diabetes, chronic liver disease or a weakened immune system.
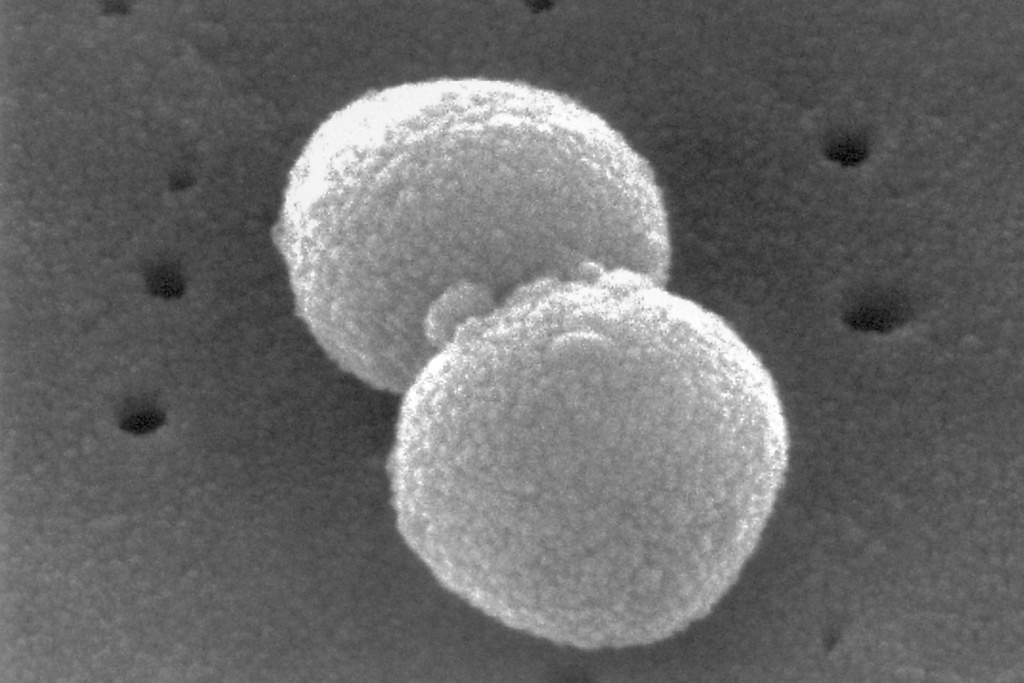
There are more than 100 known types of pneumococci bacteria, which can cause serious infections in the lungs and other parts of the body. Each year, the U.S. sees roughly 30,000 cases of invasive pneumococcal disease, which includes blood infections, brain and spine inflammation, and other illnesses. About 30% of cases are among 50- to 64-year-olds.
An adult will usually need only one pneumococcal vaccination, though some protocols advise two shots about a year apart.
The first pneumococcal vaccine was licensed in the U.S. in 1977, and since then pharmaceutical companies have been coming up with newer versions that target a dozen or more types in a single shot. Different vaccines have fallen in and out of favor, including Pfizer’s Prevnar 13, which was once a top-seller but is no longer available.
Related Articles
Striking CVS workers reach tentative agreement that boosts wages, makes health care more affordable
Second dose of COVID vaccine recommended for ages 65 and older
The mosquito-borne virus ‘triple E’ continues its spread, worrying state health officials
Newer mpox strain poses bigger risk to young women, study shows
Overdose deaths are down nationally, but up in many Western states
There are four vaccines now in use. The U.S. Food and Drug Administration this year approved the newest — Merck’s Capvaxive, which can cost around $300 a dose and protects against 21 types, including eight not included in other pneumococcal vaccines. A Merck spokesperson said it was specifically designed to help protect against the bacteria types that cause the majority of severe disease in adults aged 50 and older.
The CDC advisory panel in June recommended the vaccine as an option for adults at higher risk. At the time, the committee also talked about the possibility of lowering the age recommendation for older adults. They noted that illness-causing infections peak at age 55 to 59 in Black Americans — a lower age than what’s seen in white people. But the committee put off that decision until this week’s meeting.
The one committee member voting against the proposal was Dr. Jamie Loehr, who cited the changing guidance. “Pneumococcal has been a very confusing recommendation for many, many years, and it’s hard to have a new recommendation every two or three years,” he said.
The CDC website has a vaccine assessment tool that advises which shots to get, based on a number of factors. It has not yet been updated for the new recommendation.
The Associated Press Health and Science Department receives support from the Howard Hughes Medical Institute’s Science and Educational Media Group. The AP is solely responsible for all content.
