By Lauran Neergaard and Carla K. Johnson | Associated Press
The first person to receive a heart transplant from a pig has died, two months after the groundbreaking experiment, the Maryland hospital that performed the surgery announced Wednesday.
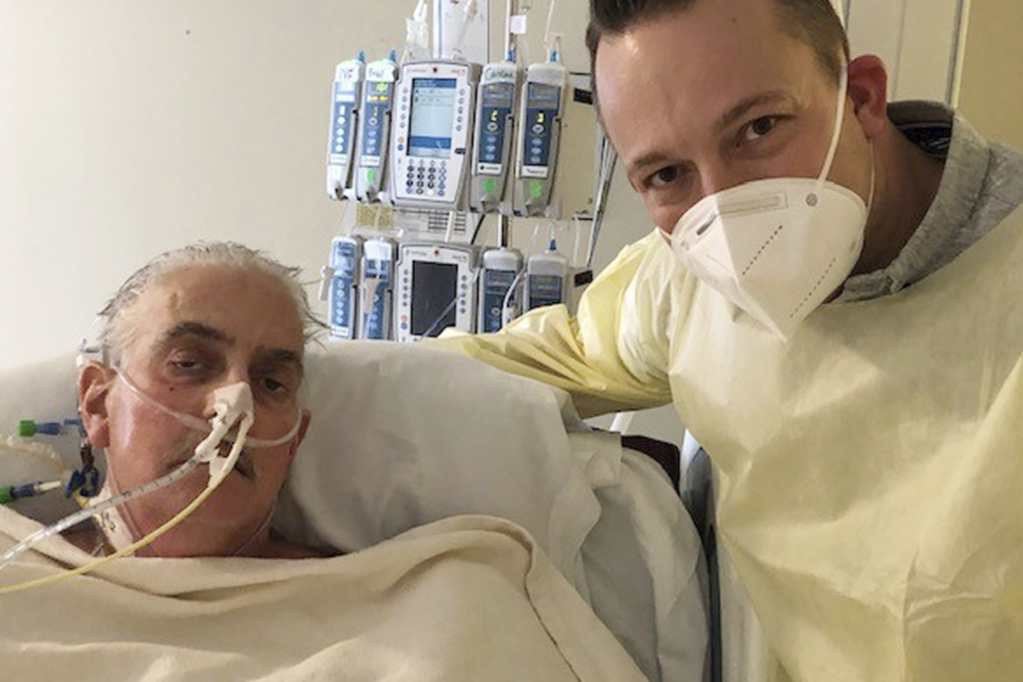
David Bennett, 57, died Tuesday at the University of Maryland Medical Center. Doctors didn’t give an exact cause of death, saying only that his condition had begun deteriorating several days earlier.
Bennett’s son praised the hospital for offering the last-ditch experiment, saying the family hoped it would help further efforts to end the organ shortage.
“We are grateful for every innovative moment, every crazy dream, every sleepless night that went into this historic effort,” David Bennett Jr. said in a statement released by the University of Maryland School of Medicine. “We hope this story can be the beginning of hope and not the end.”
Doctors for decades have sought to one day use animal organs for life-saving transplants. Bennett, a handyman from Hagerstown, Maryland, was a candidate for this newest attempt only because he otherwise faced certain death — ineligible for a human heart transplant, bedridden and on life support, and out of other options.
After the Jan. 7 operation, Bennett’s son told The Associated Press his father knew there was no guarantee it would work.
Prior attempts at such transplants — or xenotransplantation — have failed largely because patients’ bodies rapidly rejected the animal organ. This time, the Maryland surgeons used a heart from a gene-edited pig: Scientists had modified the animal to remove pig genes that trigger the hyper-fast rejection and add human genes to help the body accept the organ.
At first the pig heart was functioning, and the Maryland hospital issued periodic updates that Bennett seemed to be slowly recovering. Last month, the hospital released video of him watching the Super Bowl from his hospital bed while working with his physical therapist.
Bennett survived significantly longer with the gene-edited pig heart than one of the last milestones in xenotransplantation — when Baby Fae, a dying California infant, lived 21 days with a baboon’s heart in 1984.
“We are devastated by the loss of Mr. Bennett. He proved to be a brave and noble patient who fought all the way to the end,” Dr. Bartley Griffith, who performed the surgery at the Baltimore hospital, said in a statement.
Other transplant experts praised the Maryland team’s landmark research and said Bennett’s death shouldn’t slow the push to figure out how to use animal organs to save human lives.
“This was a first step into uncharted territory,” said Dr. Robert Montgomery of NYU Langone Health, a transplant surgeon who received his own heart transplant. “A tremendous amount of information” will contribute to the next steps as teams at several transplant centers plan the first clinical trials.
“It was an incredible feat that he was kept alive for two months and was able to enjoy his family,” Montgomery added.
The need for another source of organs is huge. More than 41,000 transplants were performed in the U.S. last year, a record — including about 3,800 heart transplants. But more than 106,000 people remain on the national waiting list, thousands die every year before getting an organ and thousands more never even get added to the list, considered too much of a long shot.
The Food and Drug Administration had allowed the dramatic Maryland experiment under “compassionate use” rules for emergency situations. Bennett’s doctors said he had heart failure and an irregular heartbeat, plus a history of not complying with medical instructions. He was deemed ineligible for a human heart transplant that requires strict use of immune-suppressing medicines, or the remaining alternative, an implanted heart pump.
Organ rejection, infections and other complications are risks for any transplant recipient. Experts hope the Maryland team quickly publishes in a medical journal exactly how Bennett’s body responded to the pig heart.
From Bennett’s experience, “we have gained invaluable insights learning that the genetically modified pig heart can function well within the human body while the immune system is adequately suppressed,” said Dr. Muhammad Mohiuddin, scientific director of the Maryland university’s animal-to-human transplant program.
One next question is what evidence, from Bennett’s experience and some other recent experiments with gene-edited pig organs, may persuade the FDA to allow a clinical trial — possibly with an organ such as a kidney that isn’t immediately fatal if it fails.
Twice last fall, Montgomery’s team at NYU got permission from the families of deceased individuals to temporarily attach a gene-edited pig kidney to blood vessels outside the body and watch them work before ending life support. And surgeons at the University of Alabama at Birmingham went a step further, transplanting a pair of gene-edited pig kidneys into a brain-dead man in a step-by-step rehearsal for an operation they hope to try in living patients possibly later this year.
Patients may see Bennett’s death as suggesting a short life-expectancy from xenotransplantation, but the experience of one desperately ill person cannot predict how well this procedure ultimately will work, said ethics expert Karen Maschke of The Hastings Center. That will require careful studies of multiple patients with similar medical histories.
Related Articles
Cedars-Sinai and UCLA scientists discover potential new COVID-19 treatment
The Super Bowl can give you a heart attack; here’s some tips to avoid one
Transplant centers should start educating their patients now about what to expect as this science unfolds, said Maschke, who with funding from the National Institutes of Health is developing ethics and policy recommendations on who should be allowed in the first studies of pig kidneys and what they need to know before volunteering.
Pigs have long been used in human medicine, including pig skin grafts and implantation of pig heart valves. But transplanting entire organs is much more complex than using highly processed tissue. The gene-edited pigs used in these experiments were provided by Revivicor, a subsidiary of United Therapeutics, one of several biotech companies in the running to develop suitable pig organs for potential human transplant.
The Associated Press Health and Science Department receives support from the Howard Hughes Medical Institute’s Department of Science Education. The AP is solely responsible for all content.
